20.11.2024
Abstract about Paper on catalyst coated diaphragms.
Paper on catalyst-coated diaphragms and how they can be used to boost alkaline water electrolysis performance for green hydrogen production.
The abstract
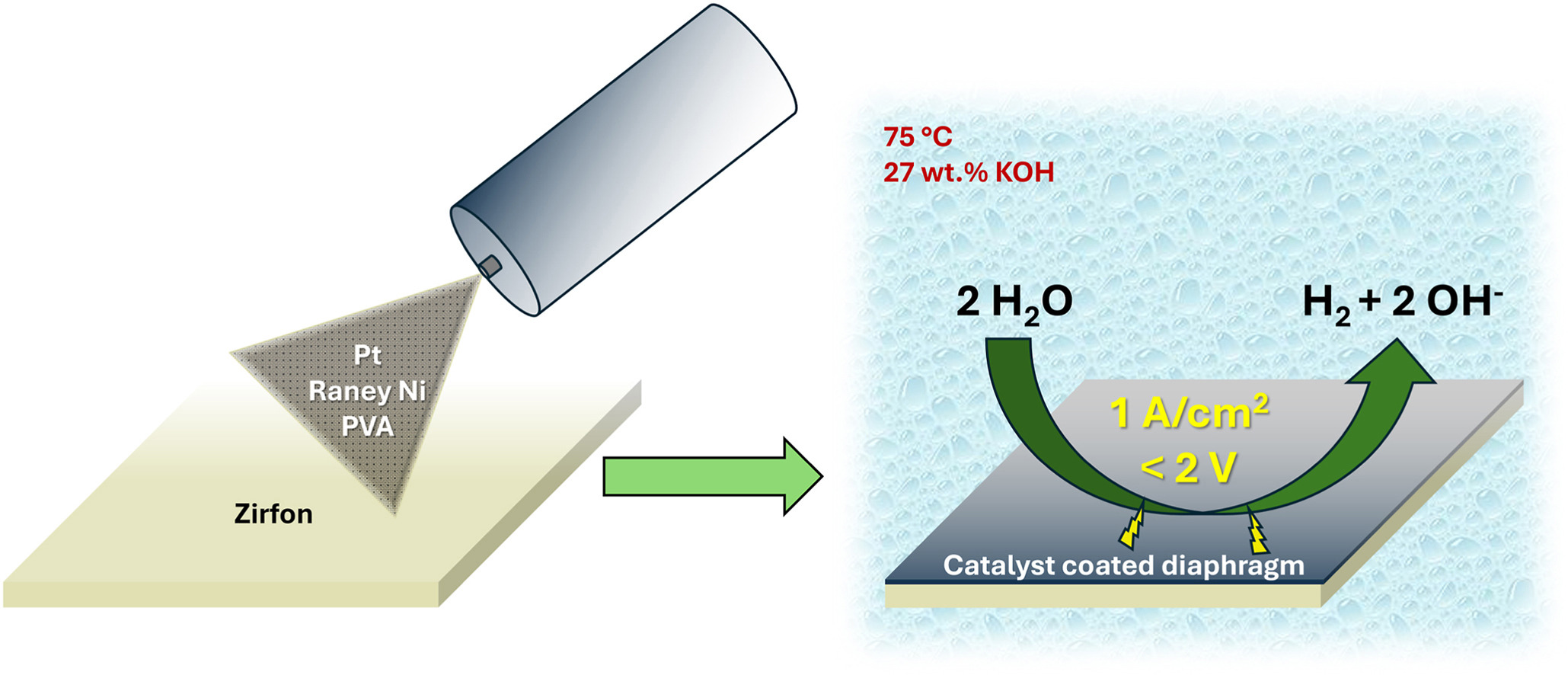
To improve alkaline water electrolysis, catalyst coated diaphragms are prepared on Zirfon UTP 500 using polyvinylalcohol as a binder and Raney Ni and Pt (20% Pt/C; nanoparticular Pt) as catalysts to enhance the hydrogen evolution reaction. The catalyst coated diaphragms are tested under close to industrial conditions to assess their electrochemical performance and stability (60+ h). Raney Ni loaded diaphragms show 200 mV cell potential reduction at low temperature, and 150 mV at high temperature throughout the entire current density range. Nanoparticulate Pt and Pt/C loaded diaphragms perform well at low current densities (<30 mA/cm2), where a overpotential reduction of 150 mV is attained. At higher current densities (>30 mA/cm2) the reduction is smaller at only 50–100 mV throughout the entire temperature range, caused by an increase in Tafel slope. Long-term measurements and SEM/EDX show that the catalyst layers become unstable after several hours of experiments.
1. Introduction
The use of fossil fuel over several centuries led to climate change encompassing the whole world as well as global dispute over finite fossil resources [[1], [2], [3], [4]]. Transitioning towards a global society free of fossil fuels requires enormous deployment of renewable energy technology, which is partially intermittent in its electricity generation profile [[5], [6], [7]]. The focus of new technological developments in the recent decades was to electrify large parts of industry, however, industrial branches exist, where electrification is uneconomical or not possible if the final products are chemicals, such as polymers or steel [[8], [9], [10], [11]]. Green hydrogen can serve as a cornerstone to store energy over long periods of time and can provide the hard to abate industry with an alternative energy carrier and feedstock [[12], [13], [14], [15], [16]].
Green hydrogen is produced by electrochemical splitting of water via the application of renewable electricity, also known as electrolysis. Alkaline water electrolysis (AWE) is the most mature electrolysis technique and utilizes strongly basic media as a highly conductive electrolyte [[17], [18], [19], [20]]. While alkaline water electrolysis is already employed on an industrial scale, further technological enhancements are desired. Alkaline electrolysers are mainly operated at low current densities due to high ohmic and polarization resistances, translating into a large physical footprint of the electrolysers. The main ohmic contributors within an electrolyser are the diaphragm and the distance between the electrodes [21]. To minimize the ohmic resistance the electrodes can be placed directly at the surface of the diaphragm, resulting in a zero-gap cell design [22]. Polarization resistances can be reduced by an appropriate choice of electrochemical catalyst enhancing the water splitting reactions [23].
A common way to incorporate catalysts into the cell is via a catalyst coated substrate (CCS) approach [[24], [25], [26], [27], [28], [29]]. Here, the catalyst is directly coated onto a conductive substrate such as Ni foam, felt, sponge, mesh, or plate. Common catalyst for AWE are Pt and Raney Ni for the hydrogen evolution reaction (HER) and FeNi layered double hydroxides (LDH) for the oxygen evolution reaction (OER) [[30], [31], [32], [33], [34], [35], [36], [37], [38], [39]].
Raney Ni electrodes are usually obtained by electrodeposition or sputtering of an alloy, followed by consecutive leaching [[40], [41], [42], [43]]. Sheela et al. showed that increasing the amount of Zn in ZnNi alloy and following Zn leaching, led to Raney Ni with a large open porous area [43]. While the Tafel slope remained the same with increasing porosity, the exchange current density increased by almost two orders of magnitude. The increase in exchange current density (when normalized by geometric area) can be connected to the increase in capacitance and electrochemical surface area (ECSA) as was shown by Gannon et al. [42] This was also displayed by Kjartansdóttir et al., who showed that when polished Ni was replaced with Raney Ni the ECSA increased by 200–500 times, due to higher roughness factors begetting higher capacitances (when the assumed geometric area was kept the same) [44]. For the HER this resulted in a reduction of the overpotential of 280–360 mV in 1 M KOH at 25 °C, with higher reductions being seen for higher ECSA and capacitances. Raney Ni may even be used for the OER via the growth of Ni oxyhydroxide films on the surface layer via electrochemical treatment. Delgado et al. synthesized highly stable Raney Ni oxyhydroxide, which did not degrade after 60 h [45]. Via impregnation of Raney Ni with Fe, Li et al. was successful in synthesizing FeNi LDH and FeNi oxyhydroxides on Raney Ni, which decreased the OER overpotential of Raney Ni from 300 to 240 mV at 10 mA/cm2 in 1 M KOH [46].
The common precious metal alternative to Raney Ni is Pt, which is also under frequent investigation for AWE: Zhang et al. conducted research on drop coated Ni foam with 20% Pt/C, resulting in loadings of 2.5 mg/cm2 [39]. They compared the Pt/C–Ni foam with a pristine foam for their HER activity in 1 M KOH at room temperature and found that the electrochemical performance was enhanced, resulting in 100 mV lower overpotentials and 60 mV/dec lower Tafel slopes. In a similar study Kong et al. performed dip coating of Ni foam into a 20% Pt/C and Nafion solution, with consecutive testing of the Pt/C–Ni foam in 1 M KOH at room temperature and current densities up to 300 mA/cm2 [47]. While they did not determine the mass loading, they found that at 10 mA/cm2 the Pt/C–Ni foam had a small overpotential of 55 mV. Based on their results Zhu et al. even argued that overpotential determination for HER using Ni(OH)2/NiO in 1 M KOH is not possible due to too high overpotentials, while they found overpotentials of less than 50 mV for Pt/C (20 wt%) at 10 mA/cm2 [48]. Liu et al. combined carbon with Ni, Pt, and PtNi and showed that the overpotential of Ni–C can be reduced by 200–220 mV for Pt–C and PtNi–C, respectively [49]. Similar trends were observed by Pei et al. when comparing the electrochemical activity of Ni(OH)2 on carbon and Pt and PtNi on carbon [50]. However, Pt based catalyst have worse catalytic performance, if the hydroxide concentration is increased, as shown by Guha et al. [51] When increasing the KOH (as well as NaOH or RbOH) concentration, the overpotential at 2 mA/cm2 increased gradually, as did the Tafel slope.
While the CCS approach is commonly used for AWE, another alternative pathway is a catalyst coated membrane (CCM) approach, originating from the proton exchange membrane (PEM) and anion exchange membrane (AEM) water electrolysis techniques [[52], [53], [54], [55], [56], [57]]. For a CCM, the catalyst, together with a binder, is directly applied to the membrane, forming a cohesive catalytic layer on the surface of the membrane. A CCM ensures a zero gap configuration of the cell, as the water splitting is occurring directly at the membrane-electrode interface. For AWE only a few studies have investigated catalyst coating of the separator. Since AWE does not employ membranes with ionic groups, but rather porous diaphragms, the corresponding terminology is catalyst coated diaphragms (CCD). One CCD study is available by Karacan et al., which focuses on the coating of Zirfon UTP 500 with Raney Ni and Nafion to improve the HER [58]. At 300 mA/cm2 (32.5 wt% KOH, 80 °C) they achieved a reduction in the cell potential by 270 mV for the best performing CCD with a loading of 36.6 ± 4.0 mg(NiAl)/cm2. The catalyst to Nafion ratio employed was 42:1. After a 1000 h test of the diaphragm, the authors observed an increase in cell potential of 60 mV at 300 mA/cm2 (60 μV/h), demonstrating a reasonable long term stability.
Nafion might not be the most optimal binder, as it was designed for acidic conditions with negatively charged surface groups, in order to facilitate proton transport. As a perfluorinated binder it may in the future be treated as a material that causes potential health concerns. A possible alternative binder was explored by Kim et al., who coated a Zirfon diaphragm using polyvinyl alcohol (PVA), which was afterwards crosslinked [59]. While the crosslinked PVA coating did not show any change in the electrochemical behavior, it served to reduce the hydrogen to oxygen (HTO) crossover by around ten times. However, Kim et al. did not explore the possibility of using PVA in combination with catalytic particles in a CCD approach.
Within this study we seek to optimize the performance of alkaline water electrolysers by employing a catalyst coated diaphragm approach for the HER using PVA as a polymer binder and nanoparticular metallic Pt, Pt loaded on graphitized C (20% Pt/C), and Raney Ni as catalysts. For those CCDs the performance was tested under close to industrial conditions (T = 21.3–76.6 °C; 27 wt% KOH; 0.5–2000 mA/cm2) using electrochemical measurements, where we also exceeded the commonly used industrial current density range. The stability was assessed via long-term cell testing (60+ h) and post-electrolysis SEM/EDX (scanning electron microscopy/energy-dispersive X-ray spectroscopy) measurements.
2. Materials and methods
2.1. Electrolyte preparation
KOH pellets (≥88%; VWR chemicals) were used to prepare the electrolyte (27 wt%) for the electrochemical measurements. Since Fe in the electrolyte can impact the performance of the electrolyser, the electrolyte was consistently spiked with 25 μM of Fe2(SO4)3 hydrate (≥76%, ≤24% H2O; Fluka Analytical; resulting in 50 μM electrolyte Fe) to obtain comparable Fe concentrations between experiments. The Fe concentration was monitored via ICP-OES (Inductively Coupled Plasma – Optical Emission Spectroscopy; iCAP™ PRO ICP-OES; Thermo Scientific™).
2.2. CCD preparation
PVA (Mw = 85,000–124,000 g/mol; 99+% hydrolyzed; Aldrich Chemistry) was dissolved in deionized water at 100 °C for several hours using a heated magnetic stirrer until a stock solution of 10 g/L was reached. The PVA was then diluted in an aqueous solution until a solid to liquid ratio of 0.25 g/L was obtained. To produce a Pt ink, a 50:50 ratio of deionized water with PVA to iso-propanol was used with either Pt/C (12.5 g/L; 20 wt% loading; Sigma Aldrich Co.) or nanoparticulate Pt (>200 nm; 2.5 g/L; Sigma Aldrich Co.). For Raney Ni (15–20 g/L; 50% slurry in water; Thermo scientific) a mixture of 12:1 of deionized water (with PVA) to ethanol was used. 1.0–1.5 mm glass balls were further added to the Raney Ni ink (around 1/3 of the entire bottle volume) and shaken over 24 h to crush Raney Ni agglomerates into smaller particle sizes.
The diaphragm was then placed on a heated plate (70–90 °C) and a spraying mask was placed on top of the diaphragm, which exposed only the desired CCD area. Next to the open CCD area a 1 cm2 reference metal plate was placed, which was weighed before and after the spraying to determine the catalyst loading. For the spray coating an air brush at 1.5 bar N2 pressure was used. For two Raney Ni CCDs (18.3 and 22.0 mg/cm2) stencil coating was performed, where a 100 μm thick template was used. The slurry of PVA and Raney Ni was then drawn with a stainless steel squeegee over the template with the exposed diaphragm. Afterwards the CCD was left to dry for 24+ h.
To increase the stability of the CCD, the PVA was then crosslinked similar to the procedure performed by Kim et al. [59] For Pt CCDs 1 g of terephthalaldehyde was added to 80 mL of water and 1 mL of conc. HCl. Ni CCDs (5.8; 18.3 mg/cm2) were crosslinked using 0.1 mL of acetic acid instead, to avoid too acidic conditions which could potentially dissolve the Raney Ni. The reaction was performed on a heated magnetic stirrer at 80 °C for 24 h. Afterwards the CCDs were washed with deionized water repeatedly to wash off residue terephthalaldehyde.
2.3. Electrochemical measurements
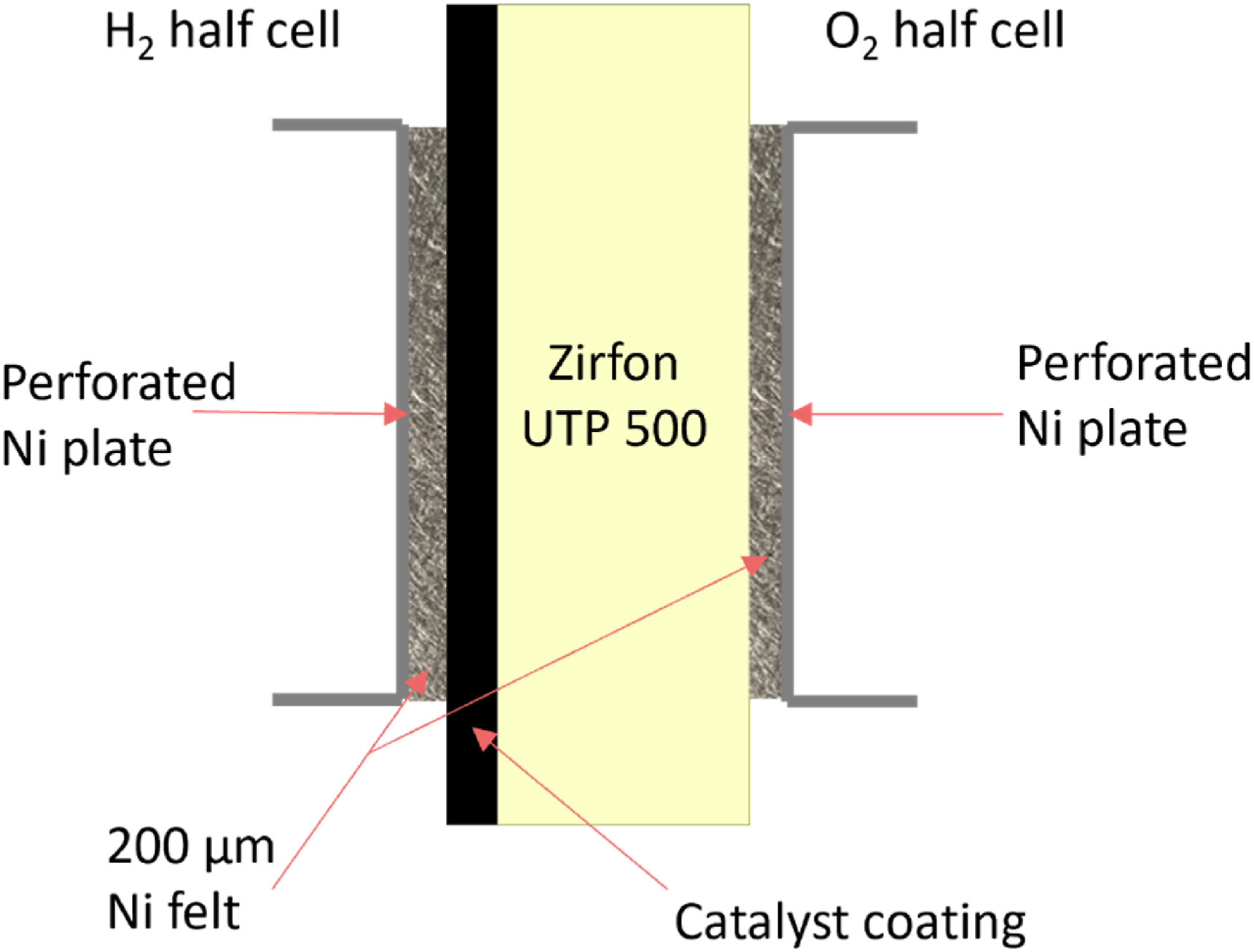
The CCDs were tested in a 3D printed flow cell (in-house design; see SI Figure S1) made from polypropylene. On both sides of the diaphragm, 200 μm porous transport layers (PTLs) (Bekaert CURRENTO® 2NI06–0.20; Ageom = 0.95x3.00 = 2.85 cm2) were placed (see Fig. 1). The current was supplied to the PTLs via Ni-201 perforated plates (A = 0.95 × 3.00 cm2 with round perforations; d = 1 mm; ∼40% open area). For assembly a torque of 2.5 N m was applied to each screw to make a zero gap configuration.
Access the full research paper HERE.
Follow EXOTHyc on Linkedin to keep posted about our latest news.